Navigating cancer immunology with the CRISPR Cancer Immune Atlas
Synopsis
- Data4Cure introduces the CRISPR Cancer Immune Atlas, featuring data from recent publications that utilize CRISPR technology to uncover key determinants of cancer immunity.
- The CRISPR Cancer Immune Atlas offers 71 screens across 10 datasets, including data from in vitro and in vivo immune challenges as well as phenotypic screens providing insights into cancer-immune interactions.
- The Atlas along with advanced features for CRISPR data analysis and visualization are now available to select partners on Data4Cure's Biomedical Intelligence Platform.
Introducing the CRISPR Cancer Immune Atlas
Recent advancements in CRISPR screening have provided new tools for immuno-oncology enabling researchers to systematically explore genes involved in immune system activation, regulation, as well as immune evasion. To leverage the growing wealth of data linking genetic perturbations to the dynamics of cancer immunity, and assist researchers in mapping the key drives and determinants of these mechanisms, Data4Cure has recently introduced the CRISPR Cancer Immune Atlas.
The Atlas comprises in vivo and in vitro data from 71 CRISPR screens from recent landmark publications covering major cancer types and spanning genome-wide CRISPR knockout, CRISPR inhibition and CRISPR activation screens. It provides a comprehensive view, including data from human and mouse cancer cell lines exposed to various immune challenges, alongside phenotypic screens.
The CRISPR screens covered by the Atlas fall under three major categories:
1) immune challenges, in which CRISPR-edited cancer cell lines are co-cultured with immune cells (e.g. CD8-positive lymphocytes or NK cells);
2) in vivo immune system challenges, in which cell lines are implanted subcutaneously into immunocompromised and immune active mice;
3) phenotypic screens, in which both CRISPR-edited T cells and cancer cells are screened for activities such as antigen presentation, cytokine secretion or proliferation.
By leveraging this wealth of integrated data, users can gain insights into how cancer cells interact with the immune system and identify genes involved in key processes including immune cell activation, immune checkpoint regulation, and antigen presentation.
Data4Cure's Biomedical Intelligence Platform for CRISPR data analysis
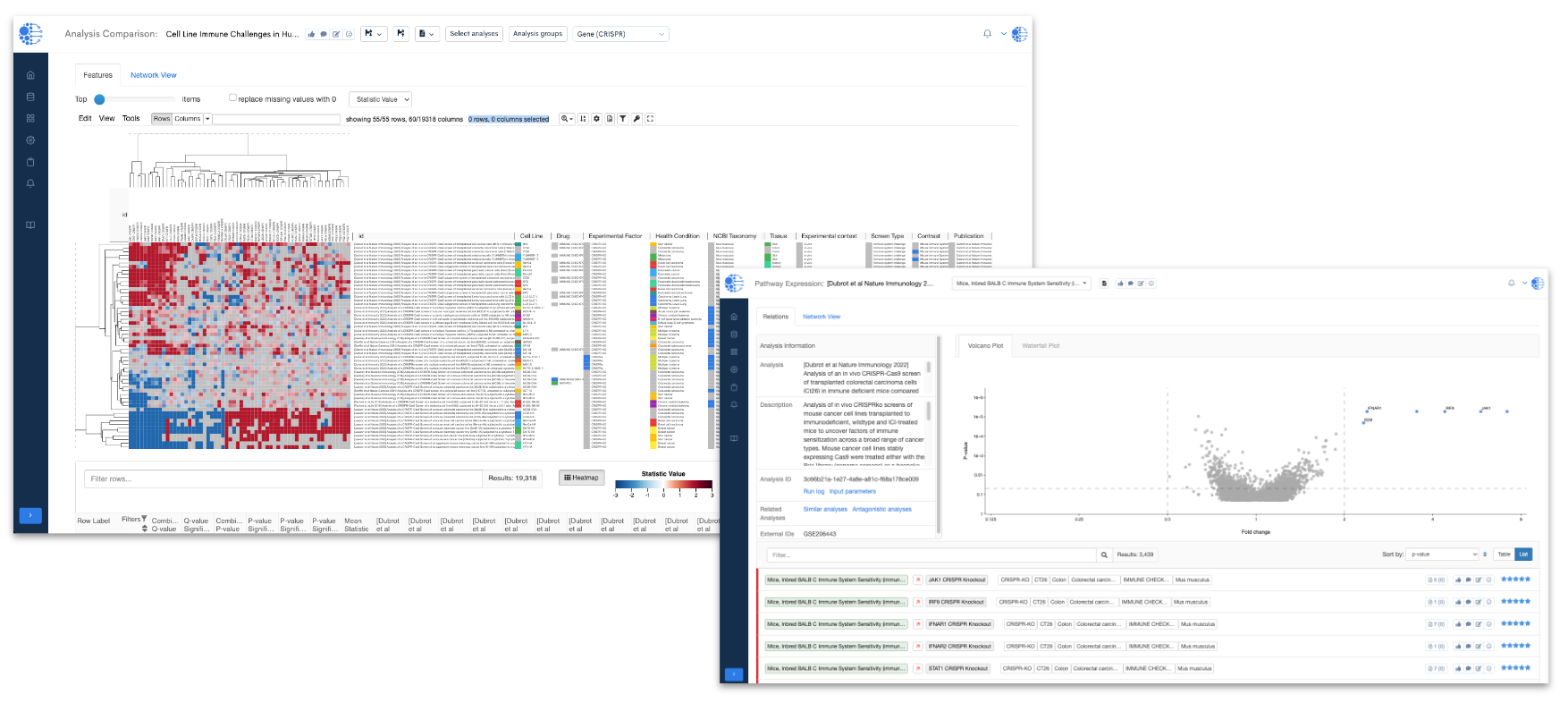
The Data4Cure Biomedical Intelligence Platform's advanced data integration, analysis and visualization features (Figure 1) are key to unlocking the potential of the CRISPR Cancer Immune Atlas.
The Biomedical Intelligence Cloud allows CRISPR data to be seamlessly integrated into with the CURIE Knowledge Graph ontologies, providing a structured framework that connects CRISPR data with relevant biological entities. This enables users to efficiently analyze these data using the Platform's Biomedical App Engine and identify gene- and pathway-level associations across CRISPR knockouts and perturbations. The Platform enables the representation of detailed guide-level perturbations along with gene-level statistics, further aiding in the exploration of critical genes and pathways involved in how cancer cells respond to immune challenges. Built-in Platform tools allow users to integrate and analyze their own data alongside the Atlas datasets. By analyzing patterns across different combinations of immune challenges, cell lines, and cancer types, users can discover and find support for new targets for cancer immunotherapy.
Genomic insights into NK cell sensitivity
Among many findings revealed by the Atlas, its integrated data highlights specific genes influencing NK cell sensitivity in several cancers including colorectal carcinoma, multiple myeloma, chronic myeloid leukemia and others, shedding light on key common factors affecting tumor response to immune challenges.
CRISPR knockout screens revealed that knockouts of NCR3LG1 and selected other genes confer resistance to NK-mediated killing, while knockouts of HLA family genes, including HLA-A and HLA-E, sensitize cells to immune challenges in different cancer types (Figure 2). By identifying such genetic factors consistently across multiple cancer types, the Atlas helps researchers build a robust list of candidate genes for further investigation in cancer immunotherapy. Researchers can then prioritize these genes based on their role in immune evasion or sensitivity, making them strong candidates for targeted therapies.
Empowering cancer immunotherapy
Data4Cure's CRISPR Cancer Immune Atlas provides a valuable resource for cancer immunotherapy research, offering insights into the genetics of cancer immunity and aiding in the discovery of new therapeutic targets. With seamless integration of CRISPR data into the Biomedical Intelligence Platform and leveraging the Platform’s advanced data analysis and visualization features, researchers gain powerful new tools to advance immunotherapy research.
If you are interested in leveraging the Data4Cure CRISPR Cancer Immune Atlas in your research, please don't hesitate to reach out to us for more information at info@data4cure.com.